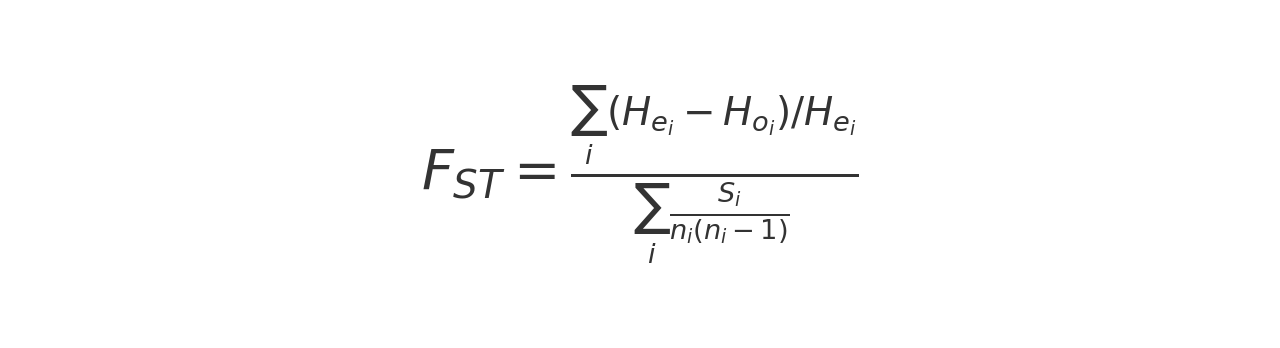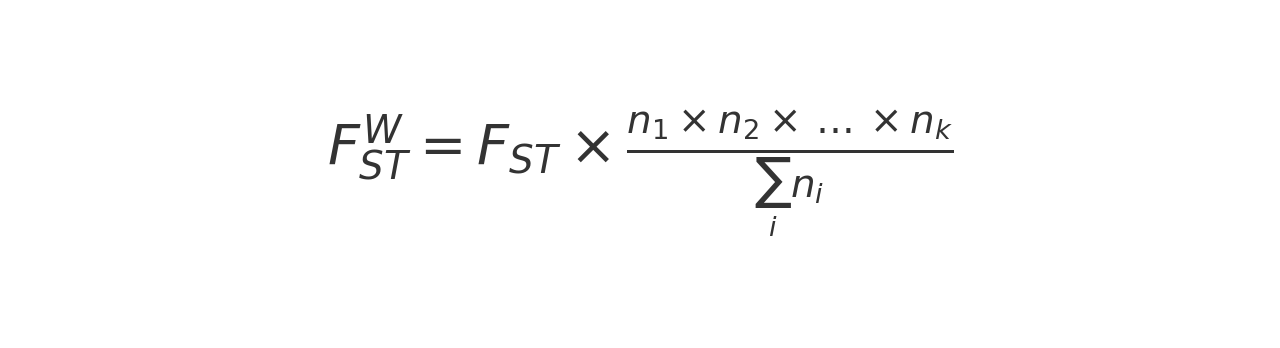popgen-evomics
Population differentiation
Fst is a statistical measure that tells us how different are two populations at the genetic level. It can be calculated for a single site, over a window (average across sites in the window), or across the entire genome.
In this session, we are going to explore how Africans and Americans differ in allele frequencies along chromosome 15. Our main task is to understand where these genetic differences occur and - possibly - why they matter. Knowing these differences but also about learning how they could affect health and diseases in various groups.
We will learn
- (a) how to calculate Fst between two populations using
vcftools - (b) how to visualize these differences
- (c) how to look for the genomic annotations at the highly diverging loci using using pangenomics
Navigate to the folder 2_fst within the mytutorial folder
cd mytutorial
cd 2_fst
(a) Fst calculation
Evaluate at which loci on chromosome 15 the Africans and Americans in the HPRC differ using vcftools. Calculate Fst index ( Weir and Cockerham’s, 1984 PMID:28563791) along the chromosome in windows of 10,000 bp:
vcftools --gzvcf /home/genomics/workshop_materials/population_genomics/chr15.pan.fa.a2fb268.4030258.6a1ecc2.smooth.reliable.vcf.gz \
--weir-fst-pop ../../metadata/afr.onlyid\
--weir-fst-pop ../../metadata/amr.onlyid \
--fst-window-size 10000 \
--out afr-amr.w10k
## --weir-fst-pop specify a file that contain lists of individuals (one per line) that are members of a population.
## The function will work with multiple populations if multiple --weir-fst-pop arguments are used.
## --fst-window-size indicate the size of the window in base pairs.
## --out afr-amr.w10k give a meaningful name to the output, I choose the population pair 'afr-amr', 'w' for windows, '10k' window size.
## It could be called 'banana', it won't matter.
This will produce:
-rw-rw-r-- 1 enza enza 866 gen 14 11:42 afr-amr.w10k.log
-rw-rw-r-- 1 enza enza 455476 gen 14 11:42 afr-amr.w10k.windowed.weir.fst
As usual, read the log file very well before looking at the results. After that look at the results:
cat afr-amr.w10k.windowed.weir.fst | column -t | less -S
## the column -t indent the columns to improve readability
You should see this:
CHROM BIN_START BIN_END N_VARIANTS WEIGHTED_FST MEAN_FST
chm13#chr15 20001 30000 212 0.032207 0.01353
chm13#chr15 30001 40000 253 0.0235088 0.0150361
chm13#chr15 40001 50000 212 0.00531486 0.00804097
chm13#chr15 50001 60000 209 0.0359234 0.0172863
The first line of the files says that: In the first 10kb from 20001 to 30000 there are 212 variants and the weighted avearge Fst is 0.3
vcftools reads the input VCF file and for each site (SNP), it calculates the allele counts and frequencies in each population. It then computes the expected heterozygosity (H_e), observed heterozygosity (H_o), and the number of polymorphic sites (S) for each population.
The FST is calculated using the following formula:
where
- i represents a population
- Hei is the expected heterozygosity in population i
- Hoi is the observed heterozygosity in population i
- Si is the number of polymorphic sites
- ni is the number of individuals in population i
The Weighted FST is then computed by multiplying the unweighted FST by the harmonic mean of the number of individuals in each population:
where:
- i is the number of populations
- ni is the number of individuals in population i.
(b) Fst along the chromosome
The following step is to create a plot of the results, offering an at-a-glance overview of the chromosome’s status. Key information for this plotting process can be found in the script ../../rscript/2.plotFst.R. You have the option to either run the script from the command line or use it as a reference to develop your own code, depending on your preference and coding style.
The scripts also writes two files with loci above a specified threshold of WEIGHTED_FST and MEAN_FST.
Rscript ../../rscripts/2.plotFst.R afr-amr.w10k.windowed.weir.fst AFR-AMR_w10k
## first argument is the Fst result file
## second argument is the title of the graph, you choose it, can be 'banana', just do not use space if multiple words
This will produce:
-rw-rw-r-- 1 enza enza 362716 gen 16 11:44 AFR-AMR_w10k.png
-rw-rw-r-- 1 enza enza 1305 gen 16 11:44 AFR-AMR_w10k.WEIGHTED_FST.high
-rw-rw-r-- 1 enza enza 1922 gen 16 11:44 AFR-AMR_w10k.MEAN_FST.high
Open png file. To view the png image, you can use Guacamole desktop or copy the file to your laptop using scp command.
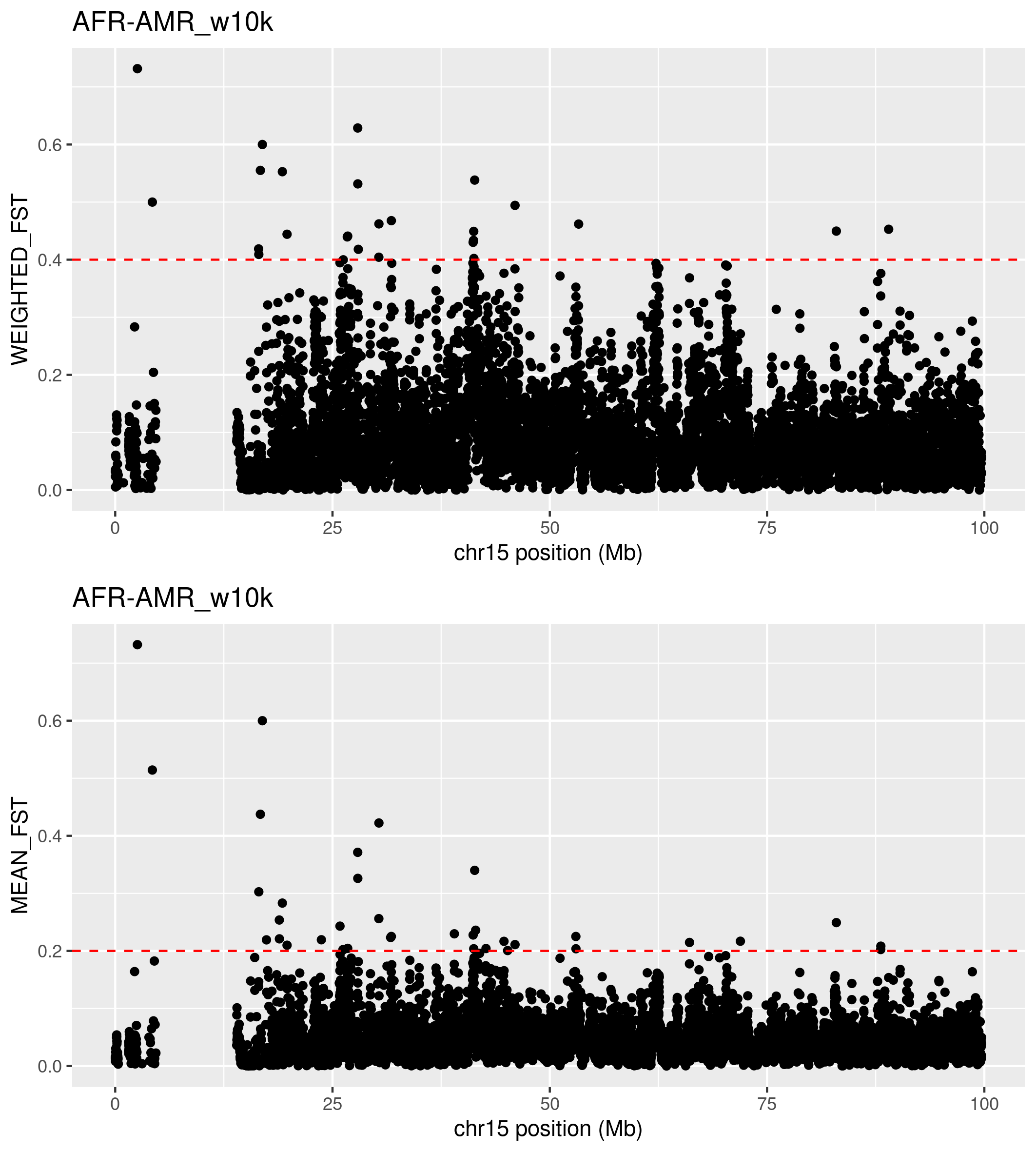
 What do you see? What do you think is the region with missing data?
What do you see? What do you think is the region with missing data?
 What do you think about all the high Fsts in the right part pf teh graph near the missing data?
What do you think about all the high Fsts in the right part pf teh graph near the missing data?
(c) Look up for highly differentiated regions
We are interested in loci with high value of Fst because they can be indicative of population-specific genetics features, as the result of genetic drift of natural selection. Filter the files with the Fsts for WEIGHTED_FST and/or MEAN_FST greather than a threshold you like. We can use awk, a programming language and command-line tool used in Unix and Unix-like systems for processing and analyzing text files, particularly useful for manipulating data, generating reports, and performing complex pattern matching.
Looking at previous plot it would be interesting to filter for WEIGHTED_FST> 0.4 or MEAN_FST>0.2:
awk 'NR==1 || $5 > 0.4' afr-amr.w10k.windowed.weir.fst > highWeightedFst.list
## NR==1 retain the header
## $5 >0.4 filters the fifth column, i.e. the WEIGHTED_FST, for values greather than 0.4
awk 'NR==1 || $6 > 0.2' afr-amr.w10k.windowed.weir.fst > highMeanFst.list
 How many regions do you select?
How many regions do you select?
It would be interesting to follow up these loci with high Fst and see which information in the genome they contain. I choose to look up a region safely far from the centromere:
CHROM BIN_START BIN_END N_VARIANTS WEIGHTED_FST MEAN_FST
chm13#chr15 82970001 82980000 70 0.449617 0.249188
If you look up the region on the UCSC Genome Browser - making sure to select the Human T2T-CHM13/hs1 reference sequence to match the coordinates - you will find that the 10kb window is upstream to the ADAMTS7P4 gene that was associated with:
- lung function in African American children of the SAGE cohortPMC:7902343
- red blood cell volume PMID:30595370
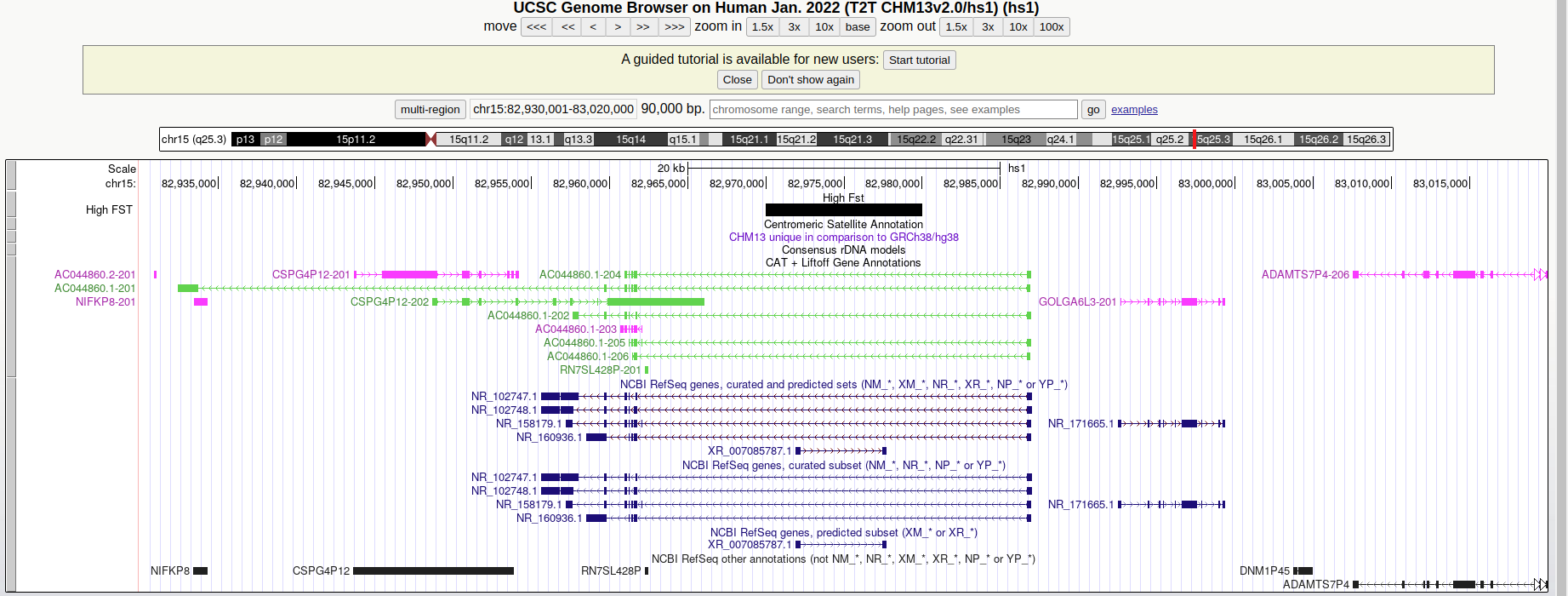
 Do you find any other interesting region?
Do you find any other interesting region?
Let’s focus on a handful of Fst peaks:
chm13#chr15 30330001 30340000
chm13#chr15 30340001 30350000
chm13#chr15 30340001 30350000
chm13#chr15 53000001 53010000
chm13#chr15 53010001 53020000
chm13#chr15 66080001 66090000
chm13#chr15 82970001 82980000
Basically there are four regions. Let’s take a look in the pangenome.
We will work through a few examples of extracting and visualizing loci. A full workup is described in these slides examining the Fst peaks vs. the HPRC chr15 pangenome graph..
1) chr15:53000001-53020000
The first peak at ~30 Mbp is embedded in a massive structural variant. That will make it harder to dissect here, so let’s go to the next one, from 53000001-53020000.
We’ll want to download a chr15-specific HRPC year 1 pangenome graph:
wget https://garrisonlab.s3.amazonaws.com/human/chr15.pan.fa.a2fb268.4030258.6a1ecc2.smooth.og.gz
This comes in the ODGI binary format, which is faster to work with than GFA for extracting regions and applying operations in odgi.
We start by pulling out the region in question:
odgi extract -i <(zcat chr15.pan.fa.a2fb268.4030258.6a1ecc2.smooth.og.gz ) \
-r chm13#chr15:53000001-53020000 -d 5000000 -t 8 -P -o - \
| odgi sort -O -i - -o hprcy1.pggb.chm13_chr15_53000001-53020000.og
This extraction grabs the region in question.
We can make some quick visualizations of the region with odgi viz:
odgi viz -i hprcy1.pggb.chm13_chr15_53000001-53020000.og -o hprcy1.pggb.chm13_chr15_53000001-53020000.og.viz.png
odgi viz -m -i hprcy1.pggb.chm13_chr15_53000001-53020000.og -o hprcy1.pggb.chm13_chr15_53000001-53020000.og.viz-m.png
odgi viz -z -i hprcy1.pggb.chm13_chr15_53000001-53020000.og -o hprcy1.pggb.chm13_chr15_53000001-53020000.og.viz-z.png
Take a look at these. What do you see in this region? Is there copy number variation (*.viz-m.* should show colors other than gray)? Is there anything strange with the orientation of paths (*.viz-z.*)?
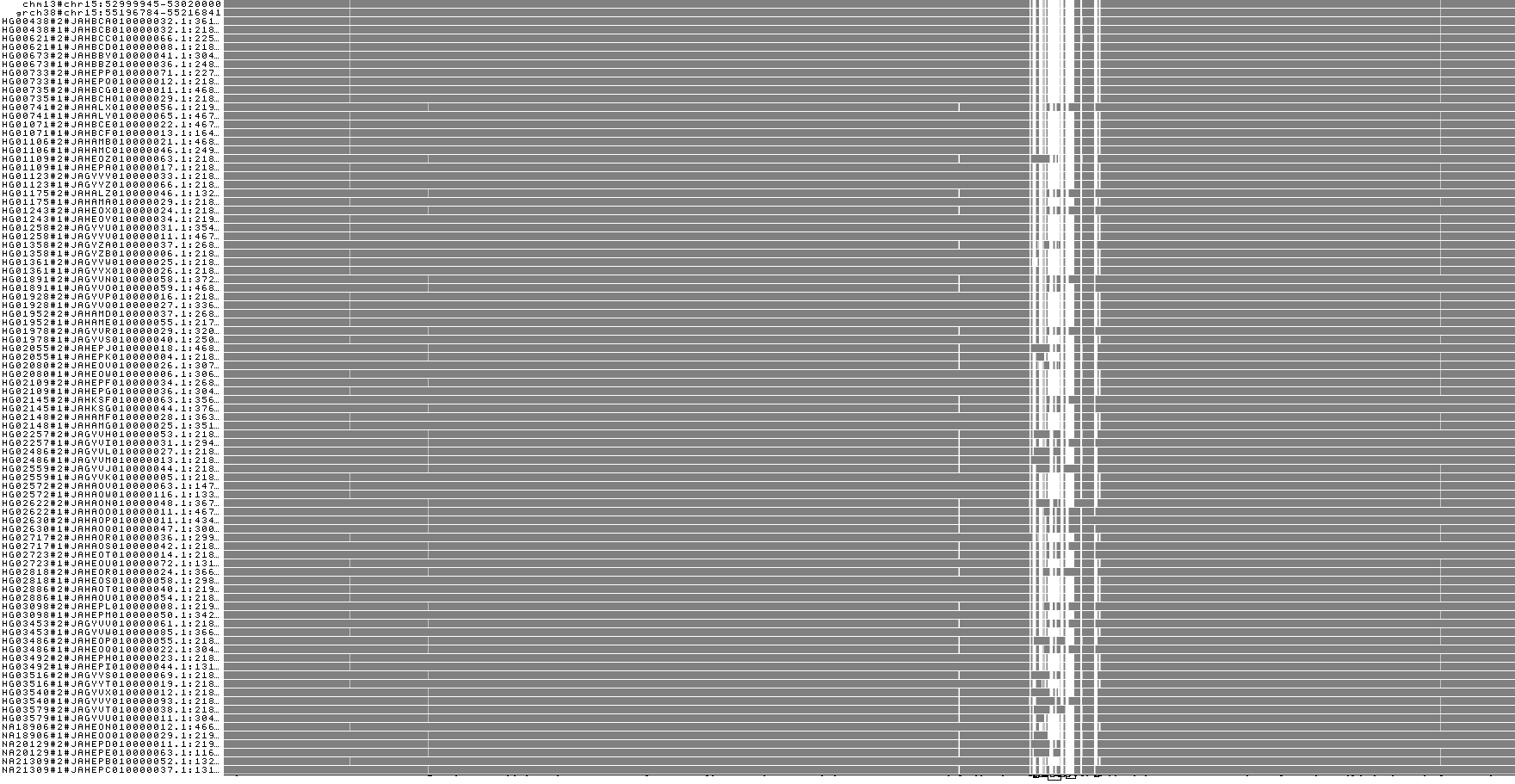
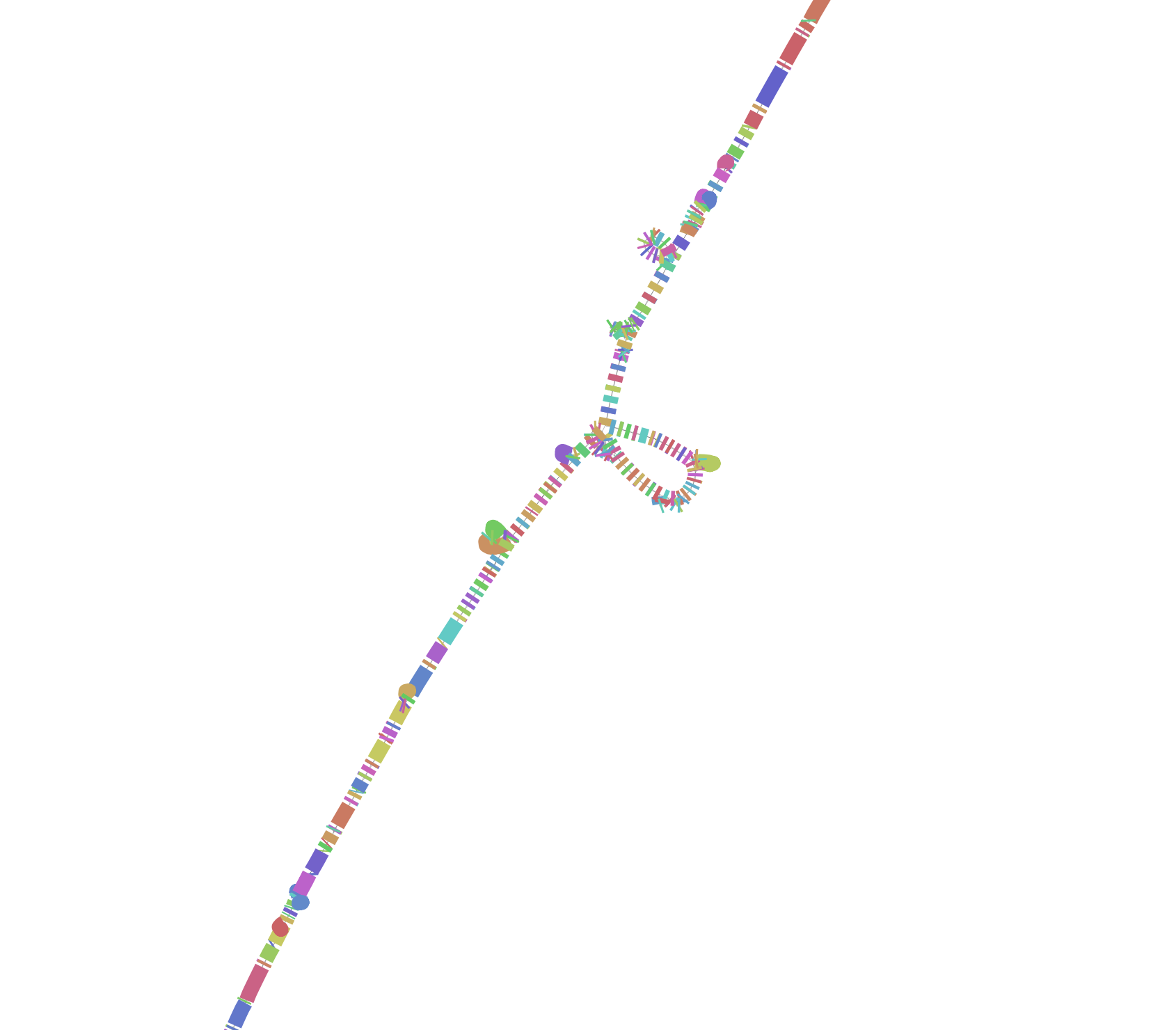
We can also open this graph in Bandage to look at the nodes and extract their sequences manually. We convert it to GFA:
odgi view -g -i hprcy1.pggb.chm13_chr15_53000001-53020000.og >hprcy1.pggb.chm13_chr15_53000001-53020000.gfa
Now this can be opened with Bandage (installed on your instance, you’ll need to open it with Guacamole).
2) chr15:66080001-66090000
On to another region!
odgi extract -i <(zcat chr15.pan.fa.a2fb268.4030258.6a1ecc2.smooth.og.gz ) \
-r chm13#chr15:66080001-66090000 -d 5000000 -t 8 -P -o - \
| odgi sort -O -i - -o hprcy1.pggb.chm13_chr15_66080001-66090000.og
# save a GFA, for later
odgi view -i hprcy1.pggb.chm13_chr15_66080001-66090000.og -g >hprcy1.pggb.chm13_chr15_66080001-66090000.gfa
# some visualizations
odgi viz -i hprcy1.pggb.chm13_chr15_66080001-66090000.og -o hprcy1.pggb.chm13_chr15_66080001-66090000.og.viz.png
odgi viz -m -i hprcy1.pggb.chm13_chr15_66080001-66090000.og -o hprcy1.pggb.chm13_chr15_66080001-66090000.og.viz-m.png
odgi viz -z -i hprcy1.pggb.chm13_chr15_66080001-66090000.og -o hprcy1.pggb.chm13_chr15_66080001-66090000.og.viz-z.png
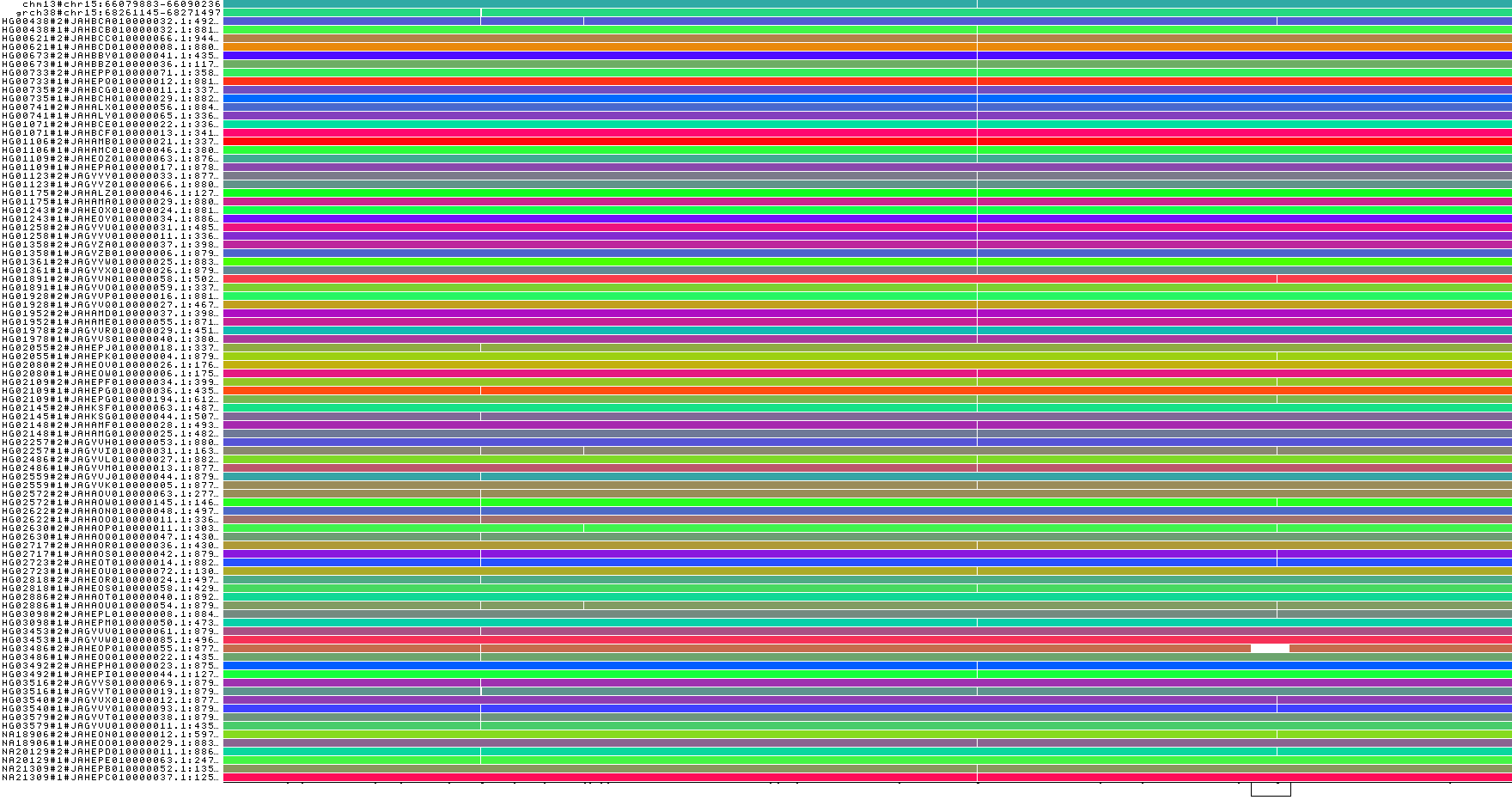
The sample order is actually correlated to population. And, it looks like there isn’t a random distribution of the indel variants across the order.
So, could we subdivide the graph into different superpopulations.
We’ll use the phyton script replace.py.
Your superpopulations are listed in this metadata file hprc_superpop.tsv.
We’ll use this to rewrite the path names and filter them by population group:
( grep -v ^P hprcy1.pggb.chm13_chr15_66080001-66090000.gfa;
grep ^P hprcy1.pggb.chm13_chr15_66080001-66090000.gfa \
| python ../../rscripts/replace.py ../../metadata/hprc_superpop.tsv | grep AFR ) \
>hprcy1.pggb.chm13_chr15_66080001-66090000.AFR.gfa
( grep -v ^P hprcy1.pggb.chm13_chr15_66080001-66090000.gfa;
grep ^P hprcy1.pggb.chm13_chr15_66080001-66090000.gfa \
| python ../../rscripts/replace.py ../../metadata/hprc_superpop.tsv | grep AMR ) \
>hprcy1.pggb.chm13_chr15_66080001-66090000.AMR.gfa
Now we can visualize each graph with odgi viz:
odgi viz -i hprcy1.pggb.chm13_chr15_66080001-66090000.AFR.gfa -o hprcy1.pggb.chm13_chr15_66080001-66090000.AFR.gfa.viz.png
odgi viz -i hprcy1.pggb.chm13_chr15_66080001-66090000.AMR.gfa -o hprcy1.pggb.chm13_chr15_66080001-66090000.AMR.gfa.viz.png
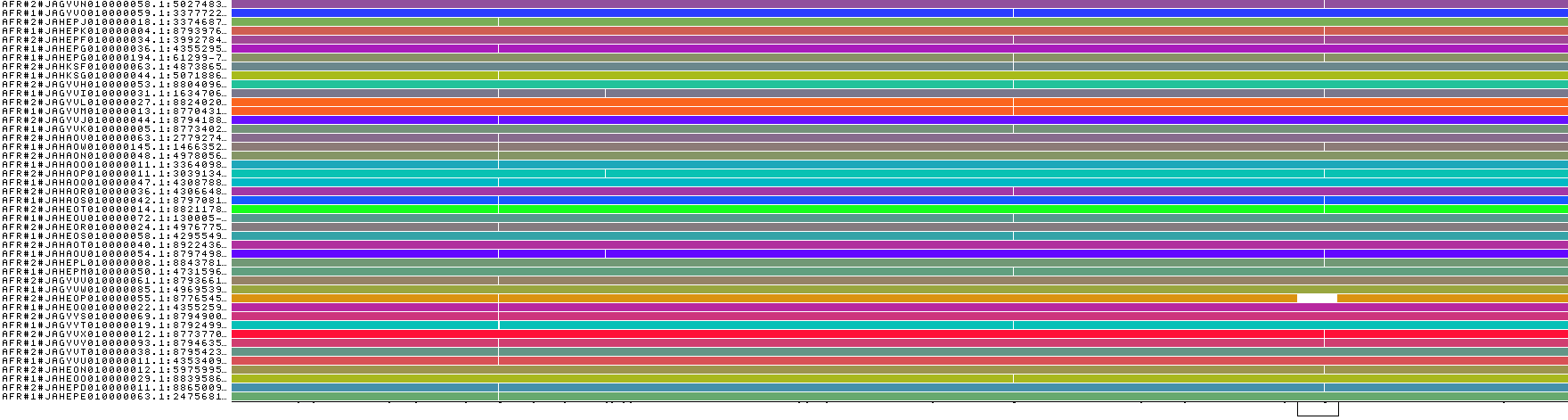
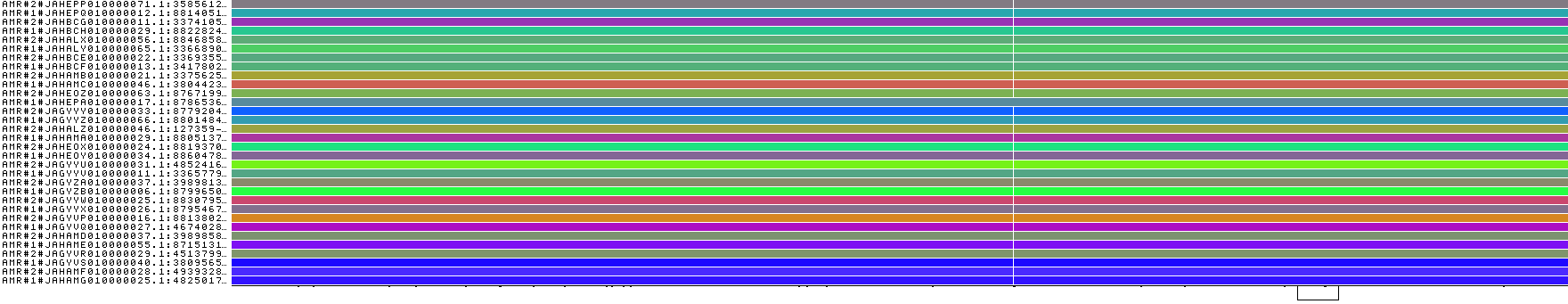
These look quite different, so that would support a high Fst.
3) chr15:82970001-82980000
Another segmental duplication! This one is big, with ~5 Mbp between the duplicated segments. A visualization of the whole chromosome with the region (expended to 200kbp) highlighted red shows where it sits:
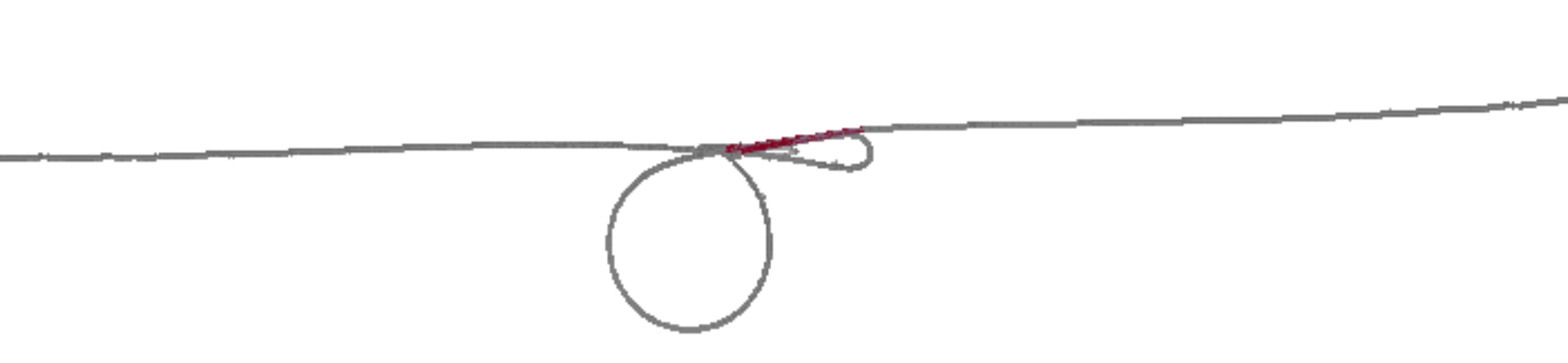
Extracting it is tricky. Here’s how you can pick up the entire region.
odgi extract -i <(zcat chr15.pan.fa.a2fb268.4030258.6a1ecc2.smooth.og.gz ) \
-r chm13#chr15:77970001-84980000 -d 5000000 -t 8 -P -o - \
| odgi sort -O -i - -o hprcy1.pggb.chm13_chr15_77970001-84980000_d5M.og
odgi viz -i hprcy1.pggb.chm13_chr15_77970001-84980000_d5M.og -m -o hprcy1.pggb.chm13_chr15_77970001-84980000_d5M.og.viz-m.png
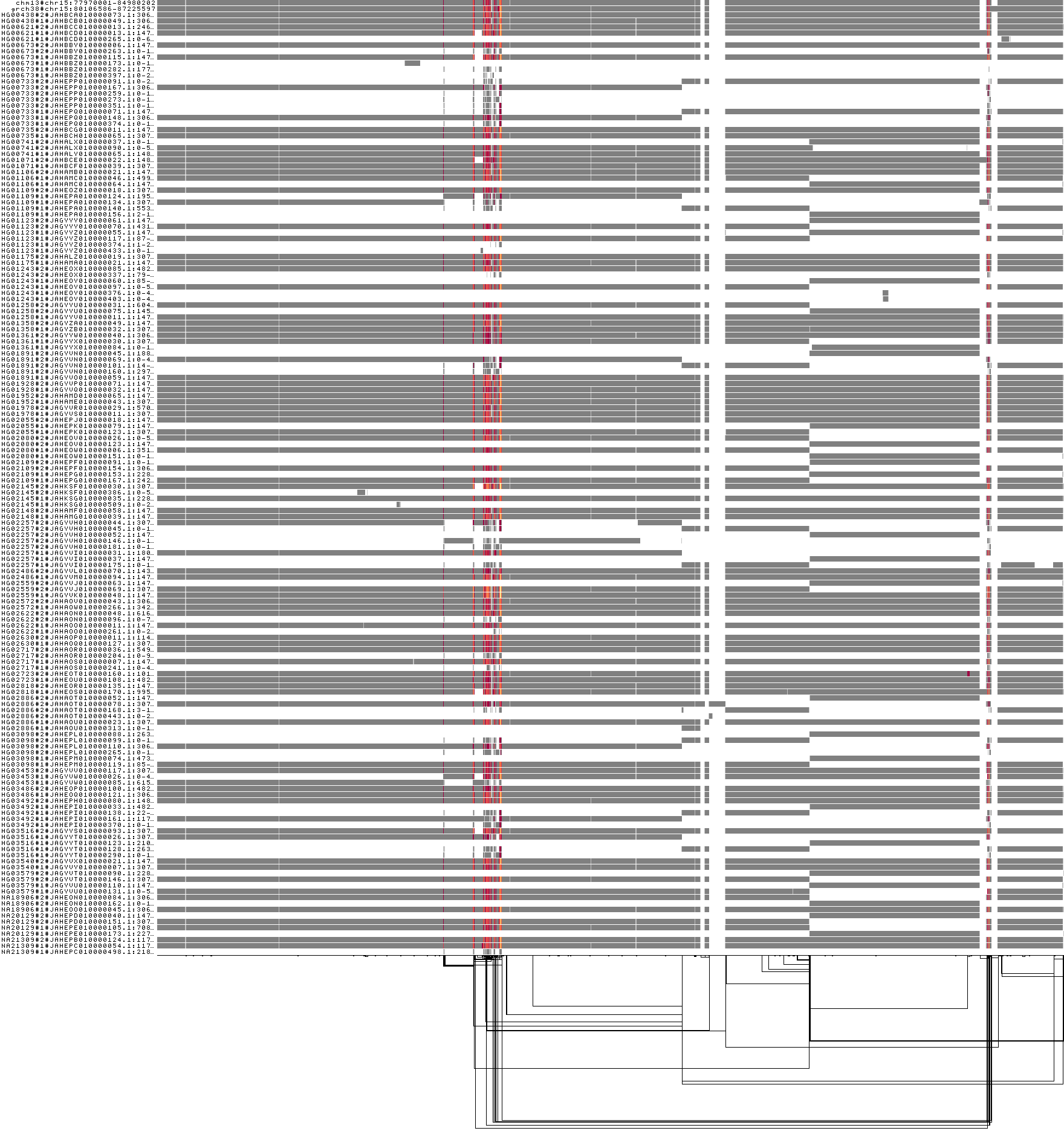
The red, orange, and yellow colors indicate higher local copy number vs. the graph (2x, 3x, 4x).
This shows that the segmental duplication region contains the Fst peak. Of course, it could be that this peak results from difficulty in establishing a correct genotyping in the locus. However, there is strong evidence that DNA evolution is accelerated in segmental duplications. There is more work to be done!